Process History
One of the oldest known fermentation products is 1, 3-Propanediol (1,3-PD) (Biebl et al., 1999). As early as 1881, August Freund reliably identified this product in a glycerol-fermenting mixed culture. It contained Clostridium pasteurianum as the active organism. It is a versatile intermediate compound that is used in the synthesis of heterocyclic products. The applications of 1, 3-propanediol is in the field of polymers, such as polyesters and polyurethanes due to the presence of two hydroxyl groups at positions 1 and 3 (Saxena et al., 2009).
In 1914, a wine-spoiling bacillus was described by Viosenet which produced the substance, although no comparable strain has been isolated. The quantitative analysis of different enterobacteria that produces 1, 3-PD through fermentation (trimethylene glycol, propylene glycol) started at the microbiology school of Delft, and was successfully continued at Ames, Iowa (Biebl et al., 1999).
Interest was shifted during the 1960’s to the glycerol attacking enzymes – glycerol and diol dehydratases in particular. These enzymes were peculiar in the requiring of coenzyme B12 (Lin, 1976). The forming of 1, 3-PD was fisrt described in 1983 as part of a process to obtain a speciality product from glycerol-excreting algae (Biebl et al., 1999).
In 1995, the Shell company announced the commercialization of a new polyester called ‘Corterra PTT’ which was based on terephthalic acid and 1, 3-PD. This changed the market situation for 1, 3-PD significantly (Saxena et al., 2009).
According to Saxena et al., (2009), the estimate share of biotechnological processes in the production of various chemicals will be around 15% by the year 2015. Therefore there is a big interest in producing bullk chemicals such as 1, 3-PD by the fermentation of microorganisms which is mainly focusing on the environmental aspects, industrial safety and renewable nature of this mode of production (Saxena et al., 2009).
Polymers that are based on 1, 3-PD are more resistant to strains and has better washfastness. 1, 3-PD was traditionally considered to be a ‘speciality chemical’, but has now been undergoing the transition into a ‘commodity chemical’ (Saxena et al., 2009).
1, 3-PD is a product from the fermentation of glycerol. It has not been found in anaerobic conversions of other organic substrates. There are only a few types of bacteria able to form it (Biebl et al., 1999). The most common of these bacteria are Citrobacter, Clostridium, Enterobacter, Klebsiella and Lactobacillus species. One of the consequences of anaerobic growth on glycerol is the generation of excess reducing equivalents in the form of reduced nicotinamide adenine dinucleotide (NADH) (Nakamura & Whited, 2003).
The conversion of glycerol to 1, 3-PD through dehydroxylation is the most exploited production strategy. There are however more strategies, such as the two-stage fermentation, in which the fermentation is carried out in two consecutive steps using either a batch or fed-batch reactor system; co-fermentation is when glycerol and other sugars are mixed to make the commercial production of 1,3 –PD less expensive; and the use of micro-aerobic conditions to minimise the chance of the formation of 3-hydroxypropionaldehyde (3-HPA), a substance that is toxic to the cells, which normally forms under aerobic conditions (Saxena et al., 2009).
Vaidyanathan et al., (2011), have used Lactobacillus reuteri in the conversion of glycerol to 1, 3-PD in a two step anaerobic process. During the first step, glycerol dehydratase catalyzes the conversion of glycerol to 3-HPA. In the second step the 3-HPA is reduced to 1, 3-PD by using a NADH-dependent oxidoreductase. The two step pathway is illustrated in figure 1.
One of the oldest known fermentation products is 1, 3-Propanediol (1,3-PD) (Biebl et al., 1999). As early as 1881, August Freund reliably identified this product in a glycerol-fermenting mixed culture. It contained Clostridium pasteurianum as the active organism. It is a versatile intermediate compound that is used in the synthesis of heterocyclic products. The applications of 1, 3-propanediol is in the field of polymers, such as polyesters and polyurethanes due to the presence of two hydroxyl groups at positions 1 and 3 (Saxena et al., 2009).
In 1914, a wine-spoiling bacillus was described by Viosenet which produced the substance, although no comparable strain has been isolated. The quantitative analysis of different enterobacteria that produces 1, 3-PD through fermentation (trimethylene glycol, propylene glycol) started at the microbiology school of Delft, and was successfully continued at Ames, Iowa (Biebl et al., 1999).
Interest was shifted during the 1960’s to the glycerol attacking enzymes – glycerol and diol dehydratases in particular. These enzymes were peculiar in the requiring of coenzyme B12 (Lin, 1976). The forming of 1, 3-PD was fisrt described in 1983 as part of a process to obtain a speciality product from glycerol-excreting algae (Biebl et al., 1999).
In 1995, the Shell company announced the commercialization of a new polyester called ‘Corterra PTT’ which was based on terephthalic acid and 1, 3-PD. This changed the market situation for 1, 3-PD significantly (Saxena et al., 2009).
According to Saxena et al., (2009), the estimate share of biotechnological processes in the production of various chemicals will be around 15% by the year 2015. Therefore there is a big interest in producing bullk chemicals such as 1, 3-PD by the fermentation of microorganisms which is mainly focusing on the environmental aspects, industrial safety and renewable nature of this mode of production (Saxena et al., 2009).
Polymers that are based on 1, 3-PD are more resistant to strains and has better washfastness. 1, 3-PD was traditionally considered to be a ‘speciality chemical’, but has now been undergoing the transition into a ‘commodity chemical’ (Saxena et al., 2009).
1, 3-PD is a product from the fermentation of glycerol. It has not been found in anaerobic conversions of other organic substrates. There are only a few types of bacteria able to form it (Biebl et al., 1999). The most common of these bacteria are Citrobacter, Clostridium, Enterobacter, Klebsiella and Lactobacillus species. One of the consequences of anaerobic growth on glycerol is the generation of excess reducing equivalents in the form of reduced nicotinamide adenine dinucleotide (NADH) (Nakamura & Whited, 2003).
The conversion of glycerol to 1, 3-PD through dehydroxylation is the most exploited production strategy. There are however more strategies, such as the two-stage fermentation, in which the fermentation is carried out in two consecutive steps using either a batch or fed-batch reactor system; co-fermentation is when glycerol and other sugars are mixed to make the commercial production of 1,3 –PD less expensive; and the use of micro-aerobic conditions to minimise the chance of the formation of 3-hydroxypropionaldehyde (3-HPA), a substance that is toxic to the cells, which normally forms under aerobic conditions (Saxena et al., 2009).
Vaidyanathan et al., (2011), have used Lactobacillus reuteri in the conversion of glycerol to 1, 3-PD in a two step anaerobic process. During the first step, glycerol dehydratase catalyzes the conversion of glycerol to 3-HPA. In the second step the 3-HPA is reduced to 1, 3-PD by using a NADH-dependent oxidoreductase. The two step pathway is illustrated in figure 1.
 |
Figure 1: Pathways of glucose and glycerol metabolism in L. reuteri. (Vaidyanathan et al., 2011) |
Lactobacillus reuteri is a heterofermentative lactic acid bacterium (LAB) which is encountered in a variety of fermented foods like sourdough, meat and dairy products. It is also a natural inhabitant of the gastro-intestinal (GI) and urogenital tract of humans and other animals (Stevens et al., 2011).
According to Viadyanathan et al., (2011), the limiting factor for the production of 1, 3-PD in L.reuteri is the growth inhibition by secret metabolites as well as toxic 3-HPA. These metabolites are produced the regenerate the cofactors such as NADH/NADPH.
L. reuteri as well as E. coli strains are grown at 37°C MRS broth and LB broth correspondingly. MRS is a substance that contains 5g yeast extract, 10g proteose peptone, 2g ammonium citrate, 5g sodium acetate, 100 mg magnesium sulphate, 50 mg manganese sulphate, 1g polysorbate 80 and 20g dextrose per litre (Vaidyanathan et al., 2011).
The inoculums for the batch reactor is grown in 150 ml MRS broth at 37°C until an OD600 of 0.8 to 1 is reached. The seed is then inoculated into a batch reactor and filled to 60% of its volume with MRS medium that contains erythromycin and glycerol
(278mM). The glucose to glycerol ratio is chosen at 1:2.5 (Tobajas et al., 2009). The fermentation is carried out at 37°C and 250 rpm in anaerobic conditions. The pH is maintained at 5.5 by adding 1.5 M NaOH or 1.5 M H3PO4 (El-Ziney et al., 1998). To establish the anaerobic condition, the reactor is flushed with sterile nitrogen (Vaidyanathan et al., 2011).
L. reuteri produces 1, 3-PD together with 3-HPA only when glycerol is co-fermented with glucose. The formation of 3-HPA will be favoured by lower levels of glucose. Higher glucose levels will generate more NADH. This is consumed for reducing 3-HPA to 1, 3-PD. By recycling NADH that is produced during glycolysis, the glycerol will function as an electron sink (Luthi-Peng et al., 2002).
By enhancing the enzyme concentration and cofactor availability could lead to improved 1, 3-PD production. This is because the phosphoketolase pathway prevalent in L. reuteri provides an increase in NADPH over the expression of yqhD, thus having the potential to further improve the productivity of 1,3-PD (Arskold et al., 2008). Through the engineering of metabolic pathways by means of road-blocking, is a suitable method for minimizing the formation of by-products (Stevens et al., 2011).
Choice of reactor
For this case study, a fed-batch reactor was chosen. The reason is to overcome substrate inhibition. If the substrate is inhibitory, the intermediate addition of the substrate will improve the productivity of the fermentation by maintaining a low substrate concentration. It is also a beneficial method due to the fact that E. coli will grow at a maximal rate if it has an unlimited supply of glucose. It will then produce organic acids as by-products. The accumulation of these by-products will inhibit the growth of the desired product (Shuler & Kargi, 2009).
Separation process
The separation of 1, 3-PD from the fermentation broth is achieved by 3 steps: Firstly is the removal of proteins, then further concentration of the broth and finally separation of 1, 3-PD by the use of chromatography. This method makes use of fairly simple equipments which has easy maintenance compared to other methods. It also resolves the major problem of high costs of currently available methods (Saxena et al., 2009). A description of this process can be seen in figure 2.
 |
Figure 2: Schematic diagram of the process for the purification of 1, 3-propanediol from fermentation broth (Saxena et al., 2009). |
 |
| Table 1: Design equations for a fed-batch reactor (Shuler & Kargi, 2009). |
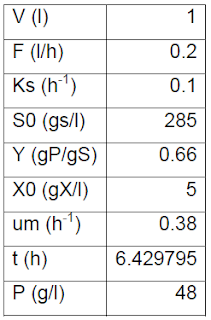 |
| Table 2: Experimental data (DROŻDŻYŃSKA et al., 2011). |
 |
| Table 3: Results from design equations. |
Initially t = 2h was used (Kaur et al., 2011), but after calculations the mass balance was set to meet the following condition by solving t; .
The average annual production of glycerol is 250 ton/year (DROŻDŻYŃSKA et al., 2011). We wish to consume 10% of this, this is equal to 684.93 kg/day or 2853.9 g/h.
After using experimental data for reactor volume (V = 1 L), the required reactor volume was calculated to meet the demand of 2853.9 gS/h, and a volume of 62.96 L was obtained.
Estimation of required equipment
To accommodate the required 62.96 L fermentation volume a 63 L reactor with a 4 mm wall thickness, will be used with the following dimensions (assuming H:D = 2:3):
.
|
 |
| Table 4: Estimation of equipment size. |


Comments
Post a Comment